The Solution
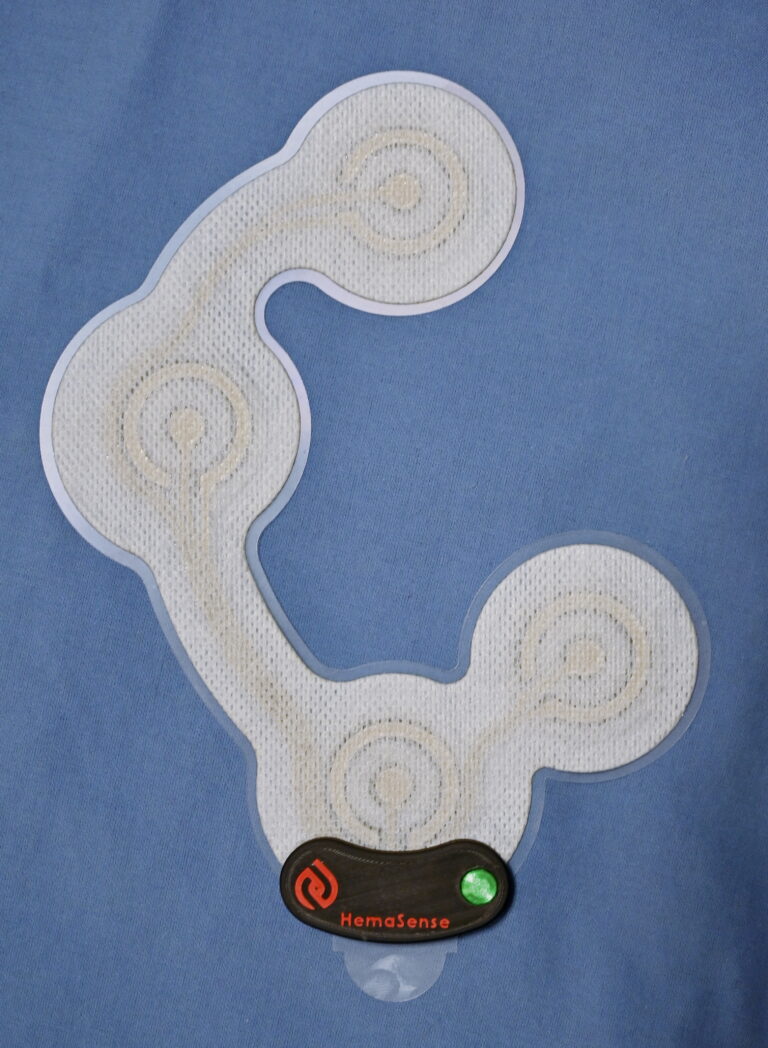
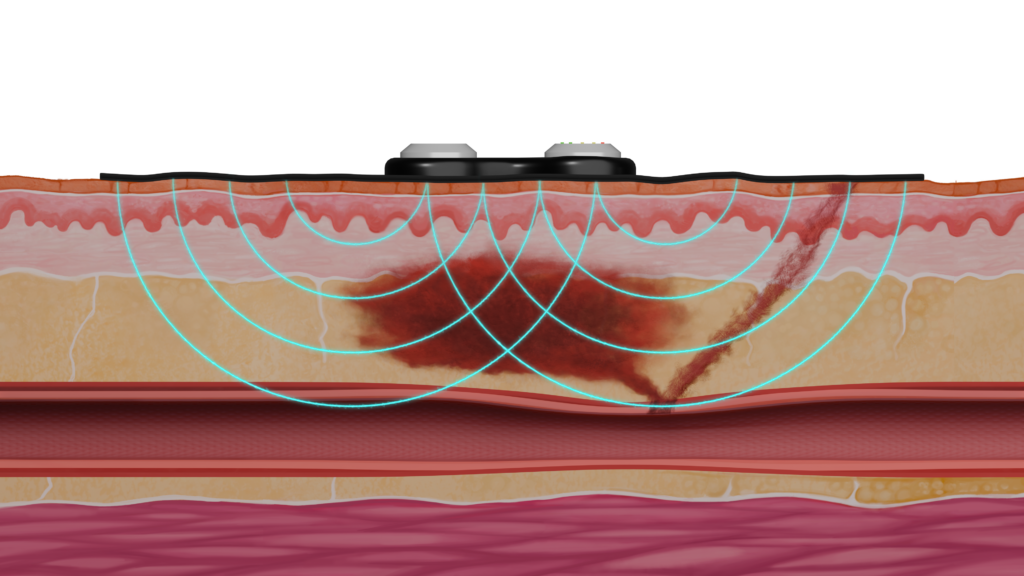
HemaSense is developing an access site monitor which is used during patient recovery to provide early detection and alert of subcutaneous bleeding events.
The HemaSense monitor seamlessly integrates with existing patient recovery workflow and enables early, less costly interventions for patients when small amounts of blood are beginning to accumulate outside the femoral vessel.
Illustrations are conceptual and do not depict the final product. This medical device is under development and not yet approved by any regulatory agency.




